Attendee Profiles
Over 1,300 clinical research professionals came together for inspiration, education, and connection in at ACRP 2025 in New Orleans. Participants represent a community of clinical research end users, influencers, and decision makers involved in clinical trial management, study conduct, and business operations and administration.
“With research constantly evolving, ACRP 2025 was a timely and refreshing deep dive into what matters most.”
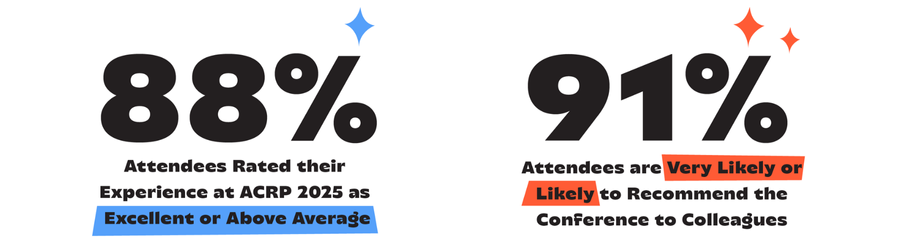
Attendee Feedback
n = 140
Attendee Demographics
By Role
By Practice Setting
n = 1,150
n = 1,157
Site Includes: Academic Medical Center/University; Clinical Study Site; Healthcare Organization/Association; Hospital; Private Medical Practice; Site Management Organization (SMO); Phase I Unit; Private Practice (Office- or Hospital-Based)
Sponsor Includes: Medical Device Company; Pharmaceutical/Biotech Company
Other Includes: Government Agency (NIH, NHS, NCI, etc.); Regulatory Agency (FDA, EMA, etc.); Training Organization; Staff Recruitment Company; Patient Recruitment Company; Institutional Review Board/Ethics Committee
Upper Management Roles Include: Billing Compliance Officer; Business Development; Director or Manager of Clinical Trial Operations; Director or Manager of Regulatory Affairs; Director of Scientific Affairs; Drug Safety Physician; Executive; Executive Management; Financial Analyst; Investigator; Medical Director; Medical Safety Officer
Support Staff Roles Include: Clinical Data Coordinator; Clinical Data Scientist; Clinical Research Coordinator; Clinical Research Nurse; Clinical Research Scientist; Data Manager; Director of Pharmacovigilance; Medical Affairs; Medical Writer; Medical Research Scientist; Monitor or Clinical Research Associate; Patient Recruiter; Pharmacist; Project Manager; Quality Control Specialist; Regulatory Specialist; Research Manager; Research Technician or Assistant; Site
Selection and Start Up; Trainer
By Experience
n = 1,241
By Focus Area
n = 405
Previous or New Attendee
ACRP Member Status
n = 140
n = 982
“The ACRP Annual Conference is a call to action for all of us in clinical research to rise above the uncertainty and shape a future rooted in innovation, inclusion, and impact. I left feeling reenergized, inspired, and hopeful for where the industry is headed.”
Attendee profiles based on ACRP 2025 participation demographic data.